Clinicians can save their patients pain and risk by following new AASLD practice guidelines advising how and when to use blood-based and imaging tests for fibrosis and steatosis, according to Richard Sterling, MD, MSc, assistant chair of research and chief of hepatology at Virginia Commonwealth University, who reviewed the guidelines recently published in Hepatology at Digestive Disease Week® (DDW) 2024.

“Chronic liver disease is very common and there’s an increased desire for and use of non-invasive liver disease assessments to reduce the pain and risks associated with liver biopsy,” Dr. Sterling says.
The guidelines and accompanying systematic reviews are the first on non-invasive liver disease assessment (NILDA) put forth by AASLD, following similar recommendations adopted by other organizations including the American Gastroenterological Association and the American College of Gastroenterology. They provide recommendations for both blood-based and imaging tests and include a section on pediatric liver disease. A separate guideline is available on NILDA for clinically significant portal hypertension.
Dr. Sterling points out several key factors of NILDA for clinicians to keep in mind:
Simple, cost-effective tests can perform just as well as proprietary tests.
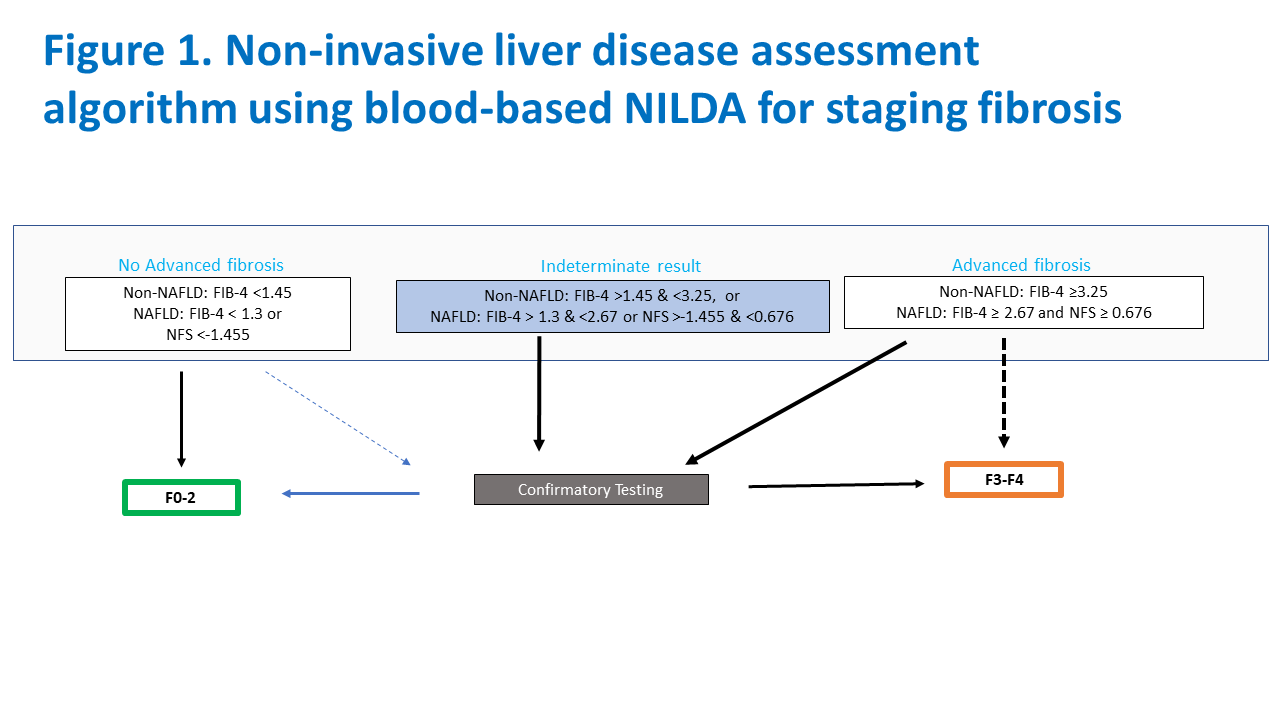
Several blood-based NILDA, such as the aspartate platelet ratio index (APRI) and fibrosis-4 index (FIB-4) can be performed from routine blood work. Dr. Sterling said that these simple blood-based tests perform similarly to more expensive, proprietary tests in identifying patients with advanced fibrosis or cirrhosis.
The guidelines broadly recommend FIB-4 for many adult patients with chronic liver disease, including those with hepatitis C virus (HCV), hepatitis B virus (HBV) and non-alcoholic fatty liver disease (NAFLD), while emerging data on metabolic dysfunction associated steatotic liver disease (MASLD) show similar accuracy.
Imaging tests, including ultrasound-based elastography and magnetic resonance elastography, are superior to blood-based NILDA and recommended, if available, to identify patients with significant to advanced fibrosis or cirrhosis. Data on the use of NILDA in pediatric populations is less robust.
Current NILDA are most useful at identifying patients on the extreme ends of liver disease.
Current NILDA perform well at identifying patients with little-to-no liver damage (to rule out) and those with advanced fibrosis or cirrhosis (to rule in), but are less useful in identifying patients with significant fibrosis (F2–F4).
“Most liver disease outcomes, such as hepatocellular carcinoma, liver failure and the need for transplant, only occur in those with advanced fibrosis,” says Dr. Sterling. “From a patient’s perspective, they want to know whether or not they have advanced fibrosis.”
However, given that many clinical trials typically focus on patients who fall in between these two extremes, the use of current NILDA may not be appropriate for clinical trial selection.
Clinical factors can impact NILDA results.
Dr. Sterling explains that a patient’s clinical situation can impact the results of both blood-based and imaging tests.
Some factors to consider include:
- Age: Several blood-based NILDA include age in their algorithms and will therefore be impacted in patients who are very young or very old.
- Platelet count: Factors such as splenectomy or immune-mediated thrombocytopenia can impact a patient’s platelet count and therefore affect several blood-based NILDA.
- Alcohol use: Alcohol use can cause several blood-based NILDA to be falsely elevated as the score will represent alcohol-based liver injury and not liver scarring.
NILDA may be less informative in treated patients.
Most of the biomarkers that inform NILDA were developed in patients who have chronic liver disease, Dr. Sterling explains. Using them after a patient has been treated for their liver disease may underestimate the extent of fibrosis because decreases in liver stiffness are often due to lower inflammation, not liver scarring.
More evidence is needed to use NILDA to monitor changes in liver damage.
Due to a lack of evidence, the guidelines do not recommend using either blood-based or imaging NILDA to monitor regression or progression of liver damage over time.
Dr. Sterling’s oral presentation, “Practice guidelines and guidance — noninvasive liver disease assessments of hepatic fibrosis and steatosis” on Saturday, May 18, at 4 p.m. EDT is part of the session “Practice Guidelines AASLD.”