As flexible endoscopes change, reprocessing techniques must also adapt to reduce the risk of infection, according to Bret Petersen, MD, professor of medicine at the Mayo Clinic, who spoke on the topic during Digestive Disease Week® (DDW) 2023.
“Inadequate or improper cleaning of reusable instruments, including flexible endoscopes, consistently ranks among the top patient safety or health care technology hazards,” Dr. Petersen said. “This is a worldwide problem that puts both patients and health care workers at risk.”
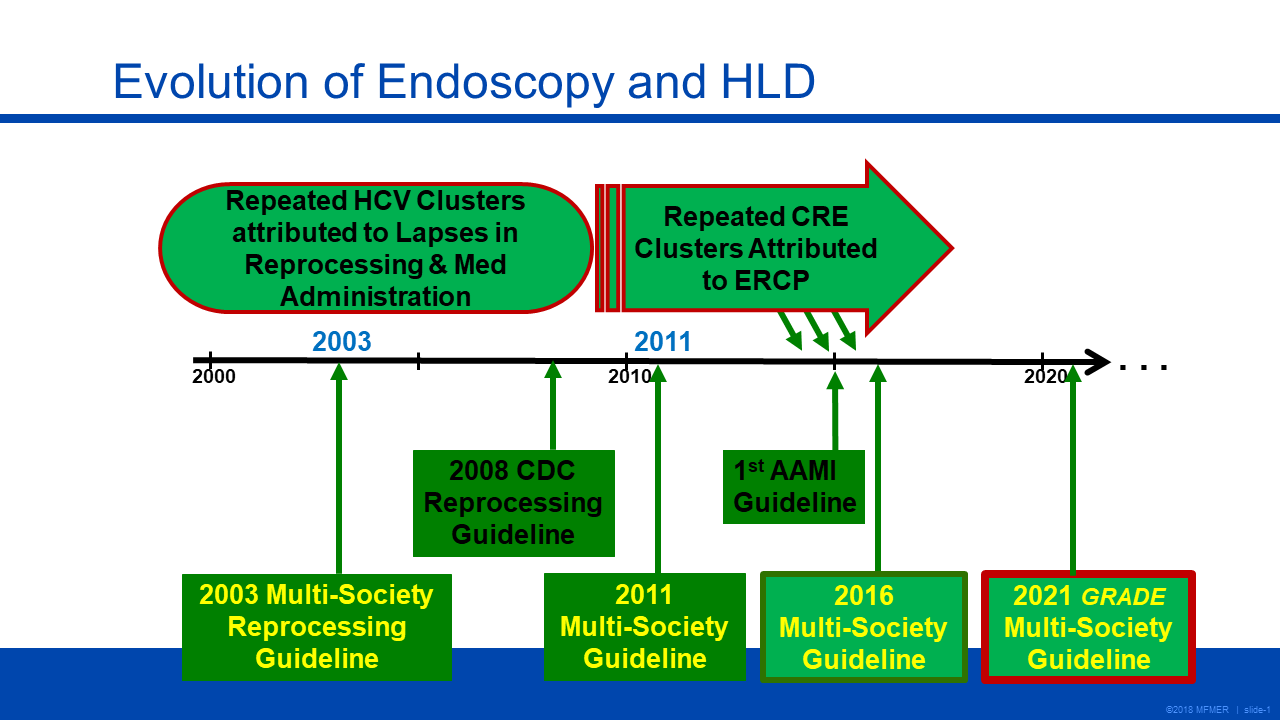
Dr. Petersen gave an overview of the 2021 “Multisociety guideline on reprocessing flexible GI endoscopes and accessories,” which was sponsored by several organizations committed to gastrointestinal endoscopy and infection control, including ASGE, AASLD, ACG and AGA.
One of the key differences between the updated guidelines and previous versions is the use of the GRADE (Grades of Recommendation Assessment, Development and Evaluation) criteria to provide recommendations on four practices that are commonly employed in endoscope reprocessing. These practices have limited data to support their use and have not generated a consensus within the field. These four unresolved questions are:
- Is there a benefit of repeat high-level disinfection compared to single high-level disinfection? The guidelines found that repeat high-level disinfection following a single cycle of washing has no added benefit in a non-outbreak setting for reducing contamination for duodenoscopes, while noting that data are limited for other types of endoscopes. The guidance did note that repetition of both washing and high-level disinfection have yielded improved results in the few studies in which this combination was assessed, but data is limited and no recommendation was made in this regard.
- Is there a benefit of ethylene oxide sterilization compared with single high-level disinfection? In general, routine sterilization of endoscopes by ethylene oxide is not recommended. The guidelines found that ethylene oxide did not reduce contamination in non-outbreak settings compared to high-level disinfection. There is data, however, to support the use of ethylene oxide sterilization to end infectious outbreaks attributed to specific instruments, and institutions may opt to employ ethylene oxide sterilization in some settings.
- What is the maximal storage time for an endoscope after it has undergone reprocessing? The guidelines found that there is not enough data to advise on a maximum storage time. They recommend that institutions develop a policy based on current data and practice needs.
- What is the efficacy of microbiologic surveillance in detecting contamination in a reprocessed endoscope? Microbiologic testing has become a recognized technique to detect bacterial contamination on reprocessed endoscopes. The guidelines recommend using standard microbiologic techniques. “This is difficult to do well,” Petersen said. “It’s not advised after every cycle or on every scope. But it is encouraged to culture every instrument periodically. For convenience, sites may choose to culture multiple instruments at the same time.”
Dr. Petersen also stressed the need for better training and realistic reprocessing schedules that take into account logistical and staffing considerations.
“This is a high-stress area for our technical staff,” Dr. Petersen said “It can be a high-pace environment, using difficult, multistep instructions. It can place a lot of stress on the least-trained members of our department. Current emphasis is on training, supervision and performance of manual cleaning steps, but we really need automated cleaning and oversight and a redesigned endoscope that is completely sterilizable.”
Dr. Petersen has been an investigator for Boston Scientific and the Danish firm Ambu, and a consultant for Pentax Medical and Olympus America. He is the immediate past president of ASGE, the primary sponsor and originator of the multisociety guideline.
Dr. Petersen gave the oral presentation, “ASGE guidelines on infection control in endoscopy” on Monday May 8, at 2:17 p.m. CDT as part of the session “ASGE & European Society of Gastrointestinal Endoscopy (ESGE)”.
If you attended DDW, your registration includes access to a recording of this session, available to watch at your convenience until May 17, 2024. Non-attendees can also purchase access to DDW On Demand.