
There is a lack of representation for racial and ethnic minorities and women in clinical trials for pancreatic ductal adenocarcinoma (PDAC), according to new data being presented at Digestive Disease Week® (DDW) 2021.
“Our findings are really the first to look at pancreatic cancer clinical trials, particularly how they have changed over time, from 2005 to 2020. And we actually looked at the different phases of clinical trials and sponsor types and how patients are represented in those subgroups,” said Kelly Herremans, MD, lead researcher on the study and surgical research fellow at the University of Florida College of Medicine. This project was conducted under the guidance of senior author Dr. Jose Trevino at Virginia Commonwealth University Massey Cancer Center.
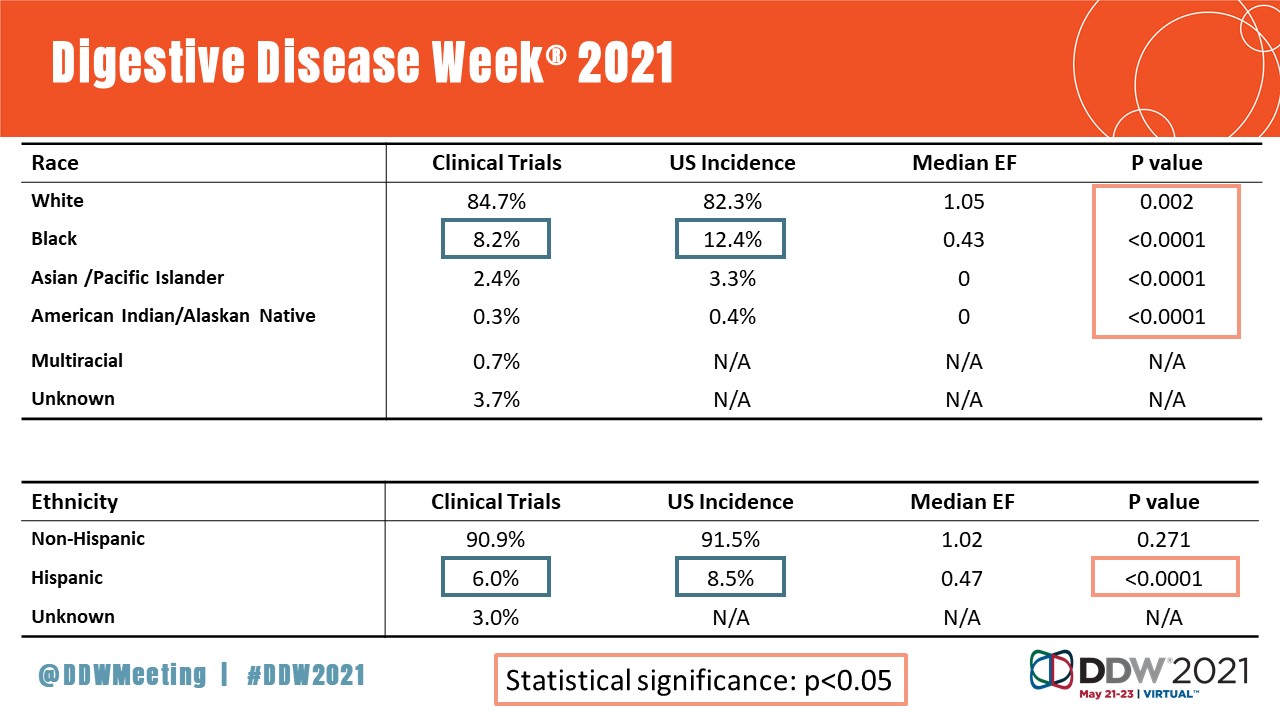
Dr. Herremans and her colleagues analyzed data from 207 PDAC clinical trials to examine clinical trial reporting and enrollment of patients by gender, race and ethnicity. While gender and race/ethnicity data were increasingly being recorded and reported over the study period, actual enrollment of these underrepresented groups lagged compared with White patients and males. Specifically:
- Hispanic (6 percent), Black (8.2 percent) and Asian/Pacific Islander (2.4 percent) individuals were notably underrepresented in PDAC clinical trials compared with U.S. population statistics.
- White individuals were overrepresented (84.7 percent) compared with their representation in the U.S. population, which is roughly 76.3 percent.
- American Indian/Alaska Native individuals made up 0.3 percent of trials compared to 1.3 percent of the U.S. population.
- Participants were about 45.2 percent female and 54.8 percent male — an underrepresentation for females and overrepresentation for males compared with the U.S. population, which is more than 50 percent female.
These trends persisted across all phases of clinical trials and were not merely reflective of discrepancies in pancreatic cancer incidence rates.
“We went back and calculated enrollment fraction, which looks at incidence of pancreatic cancer by race, and then compared that to our clinical trial participation diversity,” said Dr. Herremans. “Once we controlled for incident cases, you see underrepresentation echo throughout all of the clinical phases.”
These findings carry important treatment implications given that clinical trials — and Phase 3 trials in particular — help set the standard of cancer care for all patients and thus should be reflective of the entire cancer patient population.
Further, said Dr. Herremans, including minorities in clinical trials is imperative because certain tumor and individual biology can vary by race and ethnicity, which might affect whether a tumor responds to a study drug.
Patients of African ancestry, for instance, have been reported to have higher rates of BRCA-positive germline mutations. The advent of targeted therapies in oncology has shown that some cancer treatments may be differentially responsive to BRCA-positive tumors (e.g., PARP inhibitors).
“Although the reporting of race, ethnicity and gender has improved over time, we still haven’t reached the adequate representation of non-White, Hispanic and female patients with pancreatic cancer,” Dr. Herremans said. “We certainly need more research into why this is happening and how we can successfully fix it.”
Dr. Herremans will present data from the study, “Trials and tribulations: Diversity and inclusion in pancreatic ductal adenocarcinoma clinical trials,” abstract 901, on Sunday, May 23, at 6 p.m. EDT, during the SSAT Plenary Session: Hepatobiliary & Pancreas II.


