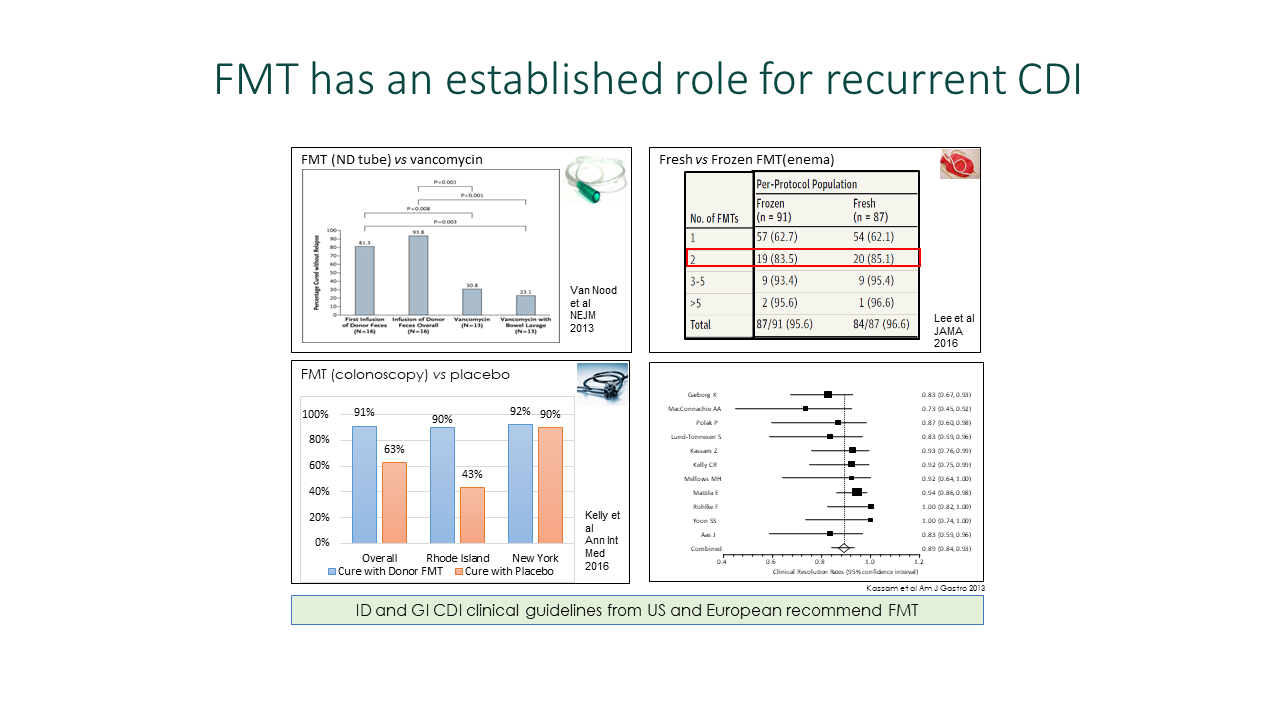
Gastroenterologists must understand the differences among fecal microbiota transplantation (FMT) products to prevent recurrent Clostridium difficile infection (CDI), according to Jessica Allegretti, MD, MPH, associate professor of medicine at Harvard Medicine School, who spoke on the topic during Digestive Disease Week® (DDW) 2024.

“The FDA approval of two new fecal microbiota products for the prevention of recurrent C. diff infection has significantly changed the treatment landscape,” said Dr. Allegretti. “FMT is one of the best strategies we have to prevent recurrent CDI, but we still face several challenges to administering these therapies.”
Dr. Allegretti compared the FMT options currently available:
- Fecal microbiota, live-jslm (Rebyota®) (FML) is a broad consortium microbiota-based therapy approved by the FDA in 2022 administered via a single rectal instillation.
- Fecal microbiota spores, live-brpk (VOWSTTM) (FMS) is a narrow consortium of live purified Firmicutes spores administered orally via capsule.
- Non-FDA approved FMT options are available from stool banks. These consist of minimally manipulated microbial communities from the stool of healthy donors. These products can currently be used to manage fulminant CDI as well as to prevent recurrent CDI without an Investigational New Drug Application.
Both FDA-approved products are indicated for prevention of recurrent CDI, but their labels do not specify how many infections a patient must have had. Dr. Allegretti advised to consider use of these products in patients with two or more CDI recurrences, though they may be appropriate earlier in some patients, such as elderly or immunocompromised patients.
Dr. Allegretti stressed that access to FML or FMS is not always straightforward. Insurance companies may deny coverage based on conflicting feedback. While companies offer patient assistance programs, these require patients to fill out paper forms, which can be difficult to manage in the era of telehealth.
Fecal microbiota transplantation is generally very safe, Dr. Allegretti said, with some patients experiencing mild gastrointestinal symptoms. Cases of recipients developing systemic infections that have sometimes proven fatal have led to minimum pathogen screening requirements by the FDA.
She provided some key considerations for gastroenterologists to choose among the three FMT options:
- Route of administration. Consider whether the patient is able to swallow capsules or has a contraindication to colonoscopy or rectal administration.
- FML and non-FDA approved FMT products are administered rectally and require that the patient be on site. In contrast, FMS is delivered directly to the patient’s home.
- Dr. Allegretti advised that physicians consider how quickly they can get the product. Insurance approval for FDA-approved products can take weeks while non-approved FMT products are often readily available and on site.
- Approval status. Patients can have strong feelings about using non-approved products, according to Dr. Allegretti. She advised that the approval, or lack thereof, of a product be included in discussions with patients.
Dr. Allegretti’s oral presentation, “How has FDA approval changed the landscape of microbiome therapeutics” on Sunday, May 19, at 10:22 a.m. EDT was part of the session “Microbiome Therapeutics: Charting the Path to Clinical Success.”