
New combination therapies, sequencing strategies and approaches to treatment modification have the potential to improve the management of inflammatory bowel disease (IBD), according to David T. Rubin, MD, professor of pathology and chief of the section of gastroenterology, hepatology and nutrition at the University of Chicago Inflammatory Bowel Disease Center. Dr. Rubin reviewed recent and ongoing head-to-head trials in IBD, including Crohn’s disease and ulcerative colitis, at Digestive Disease Week® (DDW) 2023.
According to Dr. Rubin, there have been three treatment revolutions in IBD:
- Discovering that steroids were effective and reduced mortality.
- The approval of infliximab for Crohn’s disease, which ushered in the biologic era.
- The arrival of targeted synthetic small molecules.
“We are currently in the third major wave of innovation for the treatment of IBD,” Dr. Rubin said. “New small molecule therapies offer novel mechanisms of action and a more convenient oral delivery system. They also avoid protein loss from the inflamed bowel, which has been a challenge in using monoclonal antibodies.”
Despite these advances, several unmet needs remain, including the high rate of non-response to primary treatment and the frequent loss of response. Dr. Rubin proposes several treatment approaches to increase response rates:
- Optimize the use of current therapies, including rational sequencing and dosing strategies.
- Combine therapies to increase response and remission rates.
- Incorporate rational combination therapies to address comorbid conditions that share immune pathways with IBD.
- Develop drugs with novel mechanisms of action.
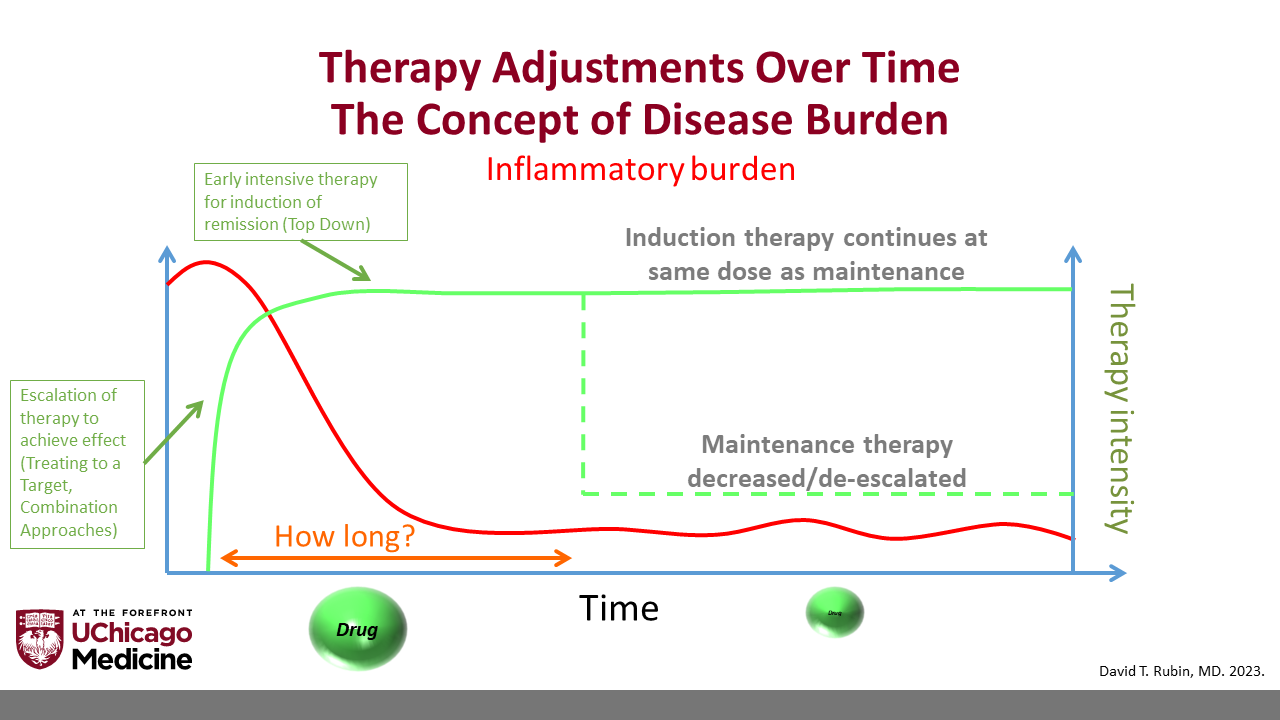
One of the key questions in treating IBD is whether and when treatment can be de-escalated, or stopped altogether, Dr. Rubin notes. He describes several scenarios in which treatment de-escalation may be appropriate:
- If the patient is receiving more treatment than is required to maintain stable disease.
- If the risks of therapy outweigh the risk of disease relapse or the benefits of disease control.
- If treatment is unaffordable.
- In a controlled, clinical research setting.
“Patients often start on intensive therapies and combinations to achieve a response,” Dr. Rubin said. “But how long do we expect them to remain on these therapies? We have a responsibility to ensure that treatment is not doing more harm than good. As the inflammatory burden decreases, it may be time to consider whether we can reduce treatment intensity to match the inflammatory state.”
Part of the way forward in developing better treatments is designing better clinical trials, Dr. Rubin asserts. He showed that patient recruitment in clinical trials has become more difficult as more FDA-approved therapies become available. Dr. Rubin’s work has shown that many patients find the screening and monitoring of clinical trials burdensome and worry about receiving a placebo or suboptimal treatment. He calls on researchers to reconsider clinical trial designs.
“IBD can have serious consequences if left un- or undertreated,” Dr. Rubin said. “We need to reconsider the use of placebo in clinical studies. It poses a risk to patients and is a detriment to trial enrollment. It is also time to consider alternative trial designs, such as adaptive trials, and novel therapeutic approaches, such as pulse therapy and modified monitoring strategies.”
Dr. Rubin’s presentation, “A look ahead: head-to-head trials, combination biologics, de-escalation, and new therapies” on Sunday, May 7, at 8:54 a.m. CDT was part of the session “AGA Farron and Martin Brotman, MD, Lecture — Transforming IBD Care 1: The Biologic Revolution — Past, Present and Future.”
If you’re attending DDW, your registration includes access to a recording of this session, available to watch at your convenience until May 17, 2024. Session captures will be released 24 hours after the session ends. Non-attendees can also purchase access to DDW On Demand to watch session recordings after DDW ends.



