With a new liver-directed therapy now available, hepatologists and gastroenterologists should focus more attention on how to best risk stratify the millions of Americans with metabolic dysfunction-associated steatotic liver disease (MASLD) and fibrosis, according to Veeral Ajmera, MD, associate professor of medicine at the University of California, San Diego. Dr. Ajmera is discussing the use of non-invasive liver disease assessments to identify these patients during Digestive Disease Week® (DDW) 2024.

“Nearly 80 million Americans have MASLD, with up to 20% having Stage 2 to 4 fibrosis,” says Dr. Ajmera. “Our goal is to find those patients because this is the population at risk for decompensation, hepatocellular carcinoma and death.”
Identifying patients with Stage 2 to 4 fibrosis has become even more important with the recent FDA approval of resmetirom, the first liver-directed therapy for metabolic dysfunction-associated steatohepatitis (MASH) with Stage 2 or 3 fibrosis. Prior to the approval, he said, clinicians focused on diagnosing cirrhosis. Having a more nuanced view of fibrosis was not as important since most patients were managed similarly.
“We have a large number of non-invasive tools available, with variable access in the community,” he says. “The test you use and the cut-offs you employ can affect how you stratify a patient in terms of risk of MASLD. The best course of action is to understand the strengths and weaknesses of all the options.”
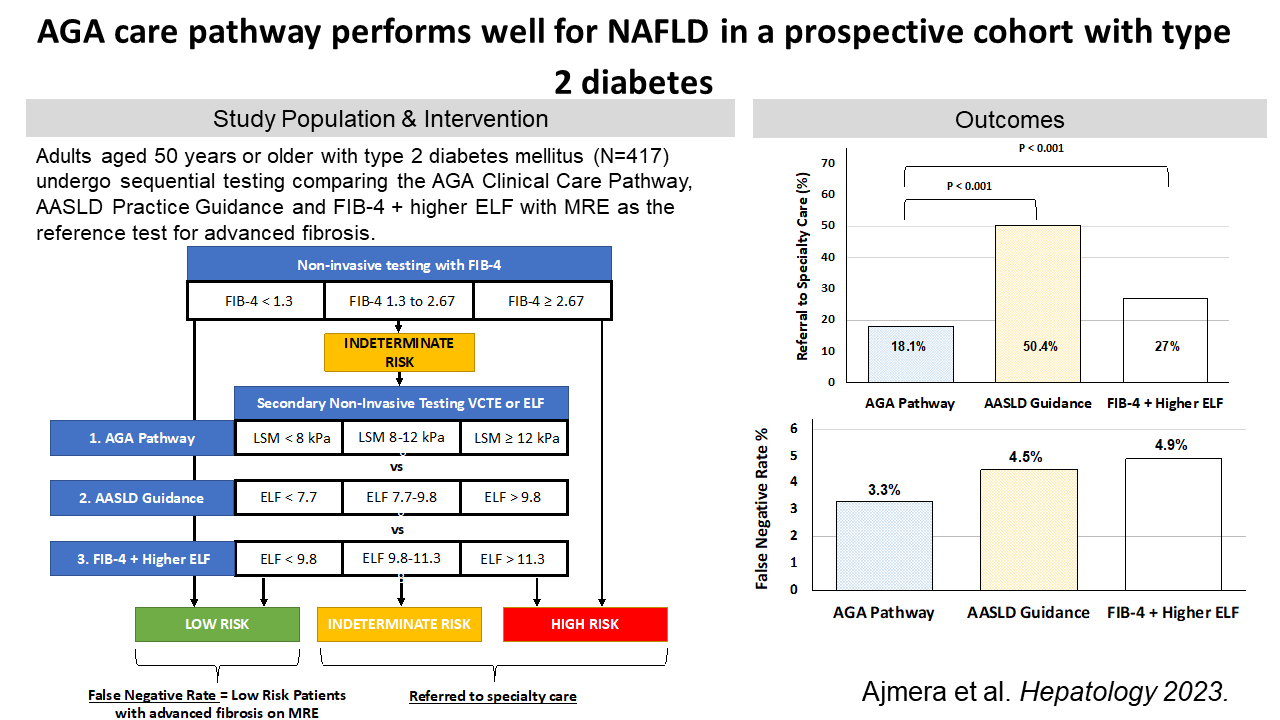
Active screening is important in high-risk populations. Advanced fibrosis and cirrhosis are particularly common in patients with diabetes and obesity. In a prospective cohort study of patients 50 years of age or older with Type 2 diabetes, 14% had advanced fibrosis and 6% had cirrhosis. In patients with additional risk factors, such as obesity, the numbers are even higher. Dr. Ajmera stresses the need for active screening in these patients to identify candidates for liver-directed therapy.
AGA and AASLD offer guidance for stratifying patients, with varying results. Both the American Gastroenterological Association (AGA) and the American Association for the Study of Liver Diseases (AASLD) have published pathways for clinicians to divide patients into low, indeterminate, or high risk based on varying liver assessments. AGA recommends the blood-based FIB-4 followed by vibration-controlled transient elastography (VCTE), while AASLD recommends FIB-4 followed by either VCTE or Enhanced Liver Fibrosis (ELFTM). Dr. Ajmera compared the two pathways in a recent prospective cohort study. He found that both pathways did well at identifying high-risk patients who needed a referral to a specialist. The AASLD pathway had a higher rate of false positives but performed better when the ELF cut-offs were increased.
The optimal way to stratify patients using non-invasive tests depends on your practice. In general, Dr. Ajmera recommends that patients be risk stratified for MASLD based on an initial simple test, such as FIB-4, particularly in lower risk primary care populations, followed by a more accurate test if the patient is deemed indeterminate risk.
However, specialists with a high-risk population may consider using a more accurate test first, such as ultrasound- or magnetic resonance imaging (MRI)-based liver stiffness measurements or ELF.
He also recommends combining tests. Some combination tests are MRI-based, which may not be available to all clinicians. Another, the FibroScan-AST (FAST) score, which uses liver elastography and aspartate aminotransferase levels, is more accessible.
“Many patients may remain in an indeterminate range, but if you have a high FAST score, you can be pretty certain the patient has a significant fibrosis and would be a good candidate for treatment,” he says.
Dr. Ajmera’s oral presentation, “Risk stratification in referrals for MASLD: GI assessment” on May 18, 2024, from 10 a.m. to 10:20 a.m. EDT is part of the session “MASLD For the Non-Hepatologist.”